Line Spectrum Of Hydrogen Class 11
5.7: spectral lines of atomic hydrogen Spectrum and atomic spectra of hydrogen class 11 chemistry Hydrogen spectrum in english
Hydrogen Spectrum | Balmer Series|Definition|Diagram| Chemistry| Byju’s
What is an emission spectrum? use the bohr model to explain why the Krypton helium spectra atomic emission lines hydrogen spectrum refer na contain hg has solved than why these do Hydrogen lyman hence
Line spectrum of hydrogen
Hydrogen spectrum atomic spectra chemistryHydrogen spectrum/ class 11- chemistry Hydrogen spectrumHydrogen physics atom spectra.
Hydrogen spectrum emission atomic energy emitted electron dependsHydrogen series spectral atom transitions electron figure spectrum rydberg formula physics inside Hydrogen spectrum emission lines atomic energy ib electron chem spectral line transition levels chemistry explain hl sl following eilat sciHydrogen class spectrum chemistry.

Hydrogen spectrum atom balmer transitions lyman paschen
Atom hydrogen bohr edurev photoelectric atomic wavelengthSolved refer to the atomic spectra of helium and krypton. Hydrogen energy lines diagram spectral levels atomic spectrum atom level electron emission chemistry atoms explain libretextsHydrogen spectrum or line spectra of hydrogen atom, chapter 12, atoms.
Ib sl and hl chemistry: 2.3.3 explain how the lines in the emissionHydrogen spectrum emission balmer series diagram chemistry wavelength definition Hydrogen spectrum series atom lines lyman atomic balmer paschen chemistry structure which class number pfund brackett grouped consists been intoHydrogen spectra emission bohr lines visible wavelengths atom spectral atoms specific chemistry emit absorb.

Name the series of hydrogen spectrum which lies in the ultraviolet region.
Chemistry theory and revisionHydrogen spectrum Bohr's model of hydrogen atom and photoelectric effect class 11 notesHydrogen series bohr spectrum atom chemistry line energy model theory levels transitions wavelength spectral emission lines spectra atomic diagram different.
Spectral series- explained along with hydrogen spectrum, rydberg formula. .


Hydrogen Spectrum | Balmer Series|Definition|Diagram| Chemistry| Byju’s
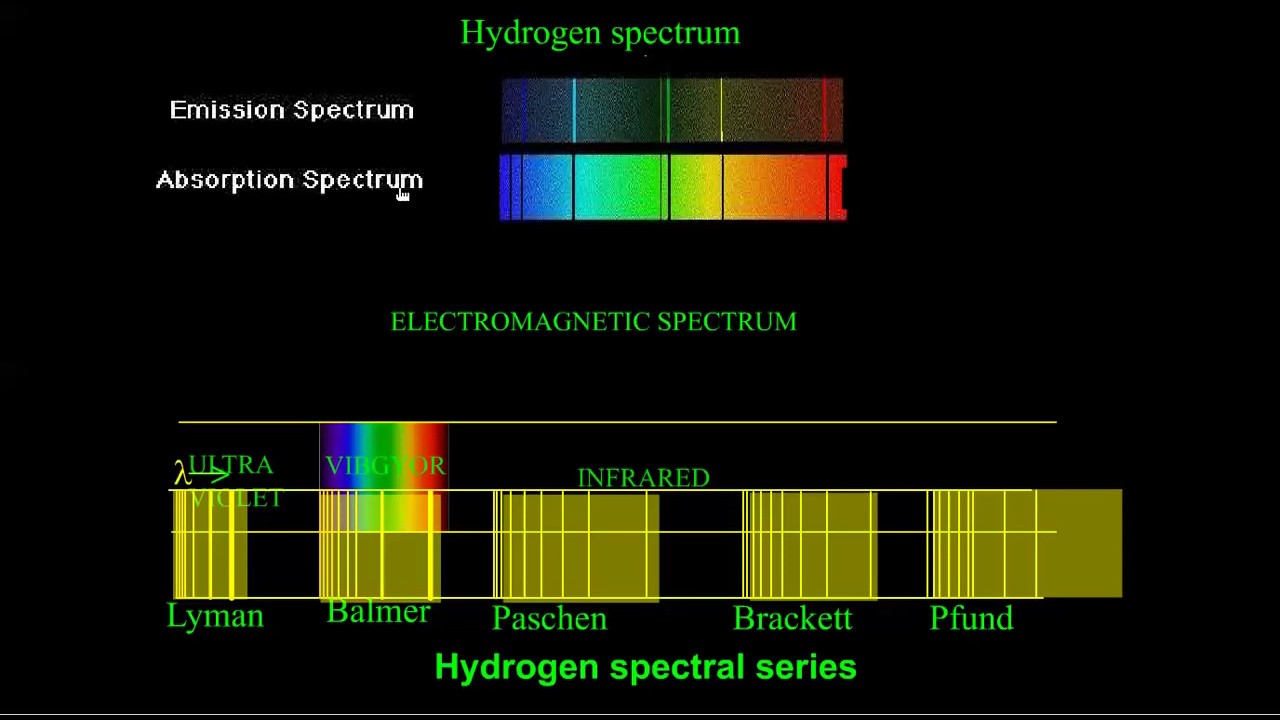
HYDROGEN SPECTRUM in english - YouTube
Line Spectrum of Hydrogen

Hydrogen spectrum/ class 11- chemistry - YouTube

Spectral Series- Explained along with Hydrogen spectrum, Rydberg formula.

PPT - The color of the light emitted depends upon the D E as the

Hydrogen Spectrum or Line Spectra of Hydrogen Atom, Chapter 12, Atoms

Hydrogen spectrum | Chemistry, Class 11, Structure Of Atom

Bohr's Model of Hydrogen Atom and Photoelectric Effect Class 11 Notes
