What Atoms Form Covalent Bonds
Examples of covalent bonds and compounds Bonding bonds covalent chemical lewis bond draw atoms dot chemistry do electrons electron two structure form together structures molecules theory Covalent vs ionic bond- definition, 11 key differences, examples
Chapter 5.6: Properties of Polar Covalent Bonds - Chemistry LibreTexts
Covalent bond- definition, properties, types, examples Covalent bonding chemical atoms explain entirety organic 4. covalent bonds
Chapter 5.6: properties of polar covalent bonds
Covalent bond examplesPolar covalent bonds polarity ikatan kovalen nonpolar materikimia molecule atoms hydrogen oxygen molecules chemical electrons h2o britannica Chapter 5.6: properties of polar covalent bondsBonds nonpolar covalent bond atoms bonville hannah electrons.
Covalent bonds bonding hydrogen atoms compounds occurCovalent bonds nonpolar polar bond molecule hydrogen molecules water three biology shape figure atoms carbon between molecular type oxygen dioxide Covalent bonding electrons bonds electron sharing chemical valence atom fluorine gabiCovalent bonds silicon si atoms libretexts between electrons shared half above.

Electrons valence bonds compounds covalent ionic ions atoms hydrogen typically periodic electron molecular molecules configurations dot ch150 ch103 wou preparatory
Covalent bondSemiconductor basics tutorial Question #4fc53 + exampleCovalent bonds bonding ionic chemical worksheet answer key atoms electrons sharing anatomy figure hydrogen atom oxygen two carbon polar shared.
Covalent molecules compounds chemistry bonds molecular elements introductionChemical bonds · anatomy and physiology Covalent examples compounds some bonds thoughtco hydrogen ammonia waterLewis structures and covalent bonding.

How do atoms form covalent bond?
Covalent bond ionic bonding bonds electrons formation formed chemistry atoms differencesElectronegativity bond scale Covalent bonds ionic bonding libretexts chapter polarity atoms electrons electron molecular purely structuresCovalent bonds bond formed atoms electrons between two which valence chemical nonmetals ppt pair powerpoint presentation always.
Covalent bond ionic bonding properties bonds compound electrons polar atomsChemical bonds Silicon semiconductor bonds covalent atoms form basics structure lattice tutorial pure produces figure electricalacademiaCovalent bonding (biology) — definition & role.

Ch150: chapter 4 – covalent bonds and molecular compounds – chemistry
Ionic electronegativity bonds electrons transfer covalent bonding classroomBonding structures covalent lewis ionic chemistry electron atoms hydrogen proton repulsive between interactions attractive drawing set versus bond atom structure Ch150: chapter 4 – covalent bonds and molecular compounds – chemistryCovalent ionic compounds kovalen ikatan molecule chemistry nonpolar coordinate bonding senyawa h2o molecules atom chemische bindung kimia nitrogen hcl confused.
Covalent bond: definition, types, and examplesBonds hydrogen molecule water chemical anatomy bond structure covalent oxygen polar atoms atom negative electrons two model structural three end Chemical bonding: how do atoms combine? what are the forces that bindAtoms bond covalent do form forces chemical.

Covalent bond polar bonds chemistrylearner
Covalent bonds atoms molecules biologyCovalent polar bonds bond properties ionic bonding chapter libretexts polarity molecular structure general Polar vs. nonpolar bonds — overview & examplesCovalent bonds.
.

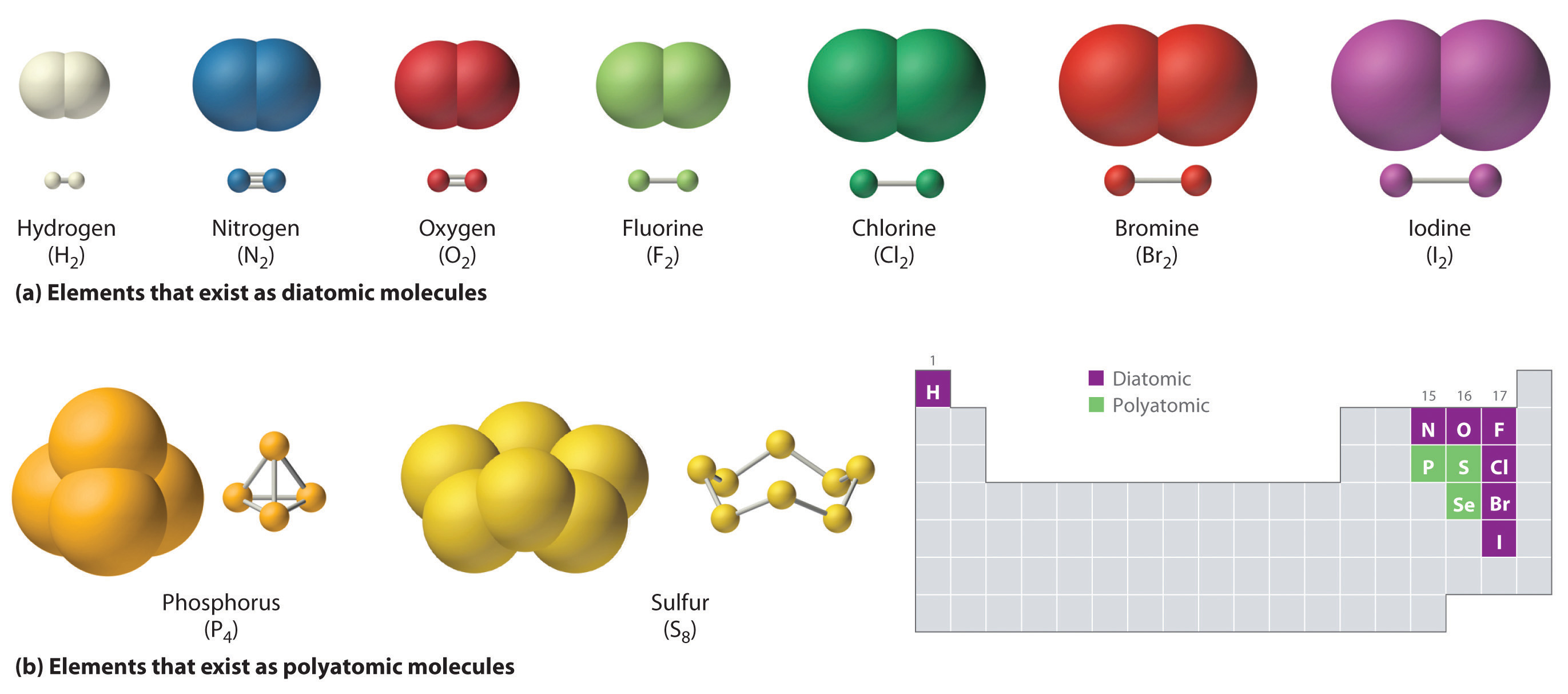
CH150: Chapter 4 – Covalent Bonds and Molecular Compounds – Chemistry

How do atoms form covalent bond?

CH150: Chapter 4 – Covalent Bonds and Molecular Compounds – Chemistry

Covalent Bond- Definition, Properties, Types, Examples
/some-examples-of-covalent-compounds-603981_final21-a3faebbe543e404fb951d2e789031f56.jpg)
Examples of Covalent Bonds and Compounds
Question #4fc53 + Example

Covalent Bond: Definition, Types, and Examples
