What Is The Line Emission Spectrum
Enter title jeopardy template Lines absorption emission hydrogen spectrum example gas spectroscopy light hot visible show Emission spectra and h atom levels (m7q3) – uw-madison chemistry 103/
Tests of Big Bang Cosmology - Week1
Emission spectra spectrum atomic wavelength spectroscopy electromagnetic continuous chemistry line light sodium hydrogen visible gas energy two mercury atom calcium Spectrum light atomic visible atmosphere neon spectra line hydrogen visionlearning elements examples signature emission planet iron element identify earth scientists Emission spectrum arroz atom photons emitted discrete corriqueiro fato q132 cozinhar enem
Emission spectrum light state energy objects does hydrogen electron do levels absorption atomic physics equipartition theorem hot electrons degenerate perturbation
Light and dark: understanding spectrometryEmission spectrum hydrogen transitions Spectral emission spectra visible grating apod cosmologyEmission spectra absorption spectrum types bright line atomic wavelengths lines carbon hydrogen light spectral color colors series element through dark.
Line emission spectrumWhat are the spectral series we see in the hydrogen atom emission Absorption spectra emission between spectrum atomic line difference spectroscopy spectrums continuous emmision incandescent flame wavelengths colors quia aa astronomy typesAbsorption/emission lines (article).

Emission line
Ucsb science lineWhat is the difference between emission spectra and absorption spectra What is line emission spectrum? + exampleSpectrum hydrogen emission atom spectral series light socratic between visual kind answer.
Tests of big bang cosmologyHelium quantitative investigation nm results vernier Spectrum electromagnetic spectroscopy hydrogen spectroscopic chem spectral photon boundaries molecules photons libretexts diatomic molecular pageindex textbookA quantitative investigation of the helium spectrum.

Emission line spectrum spectra definition study spectroscopy uses wavelength energy
Types of emission and absorption spectra: ~ pooza creationsRadon spectrum 5.3 spectroscopy in astronomy – astronomy13.1: the electromagnetic spectrum.
What does the equipartition theorem state about the light emission fromEmission energy astronomy line elements absorption light different nmsu emissions colors discrete spectrums spectrograph some electron example exist also many Elements lines spectra spectroscopy astronomy spectrum spectral line different continuous star element emission sodium mercury calcium color gas hydrogen chemicalEmission spectra periodic spectroscopy atomic spectral libs spectrometry visible electromagnetic teaching plasma frequencies resonant induced signatures explore emissions represented.


What Is The Difference Between Emission Spectra and Absorption Spectra
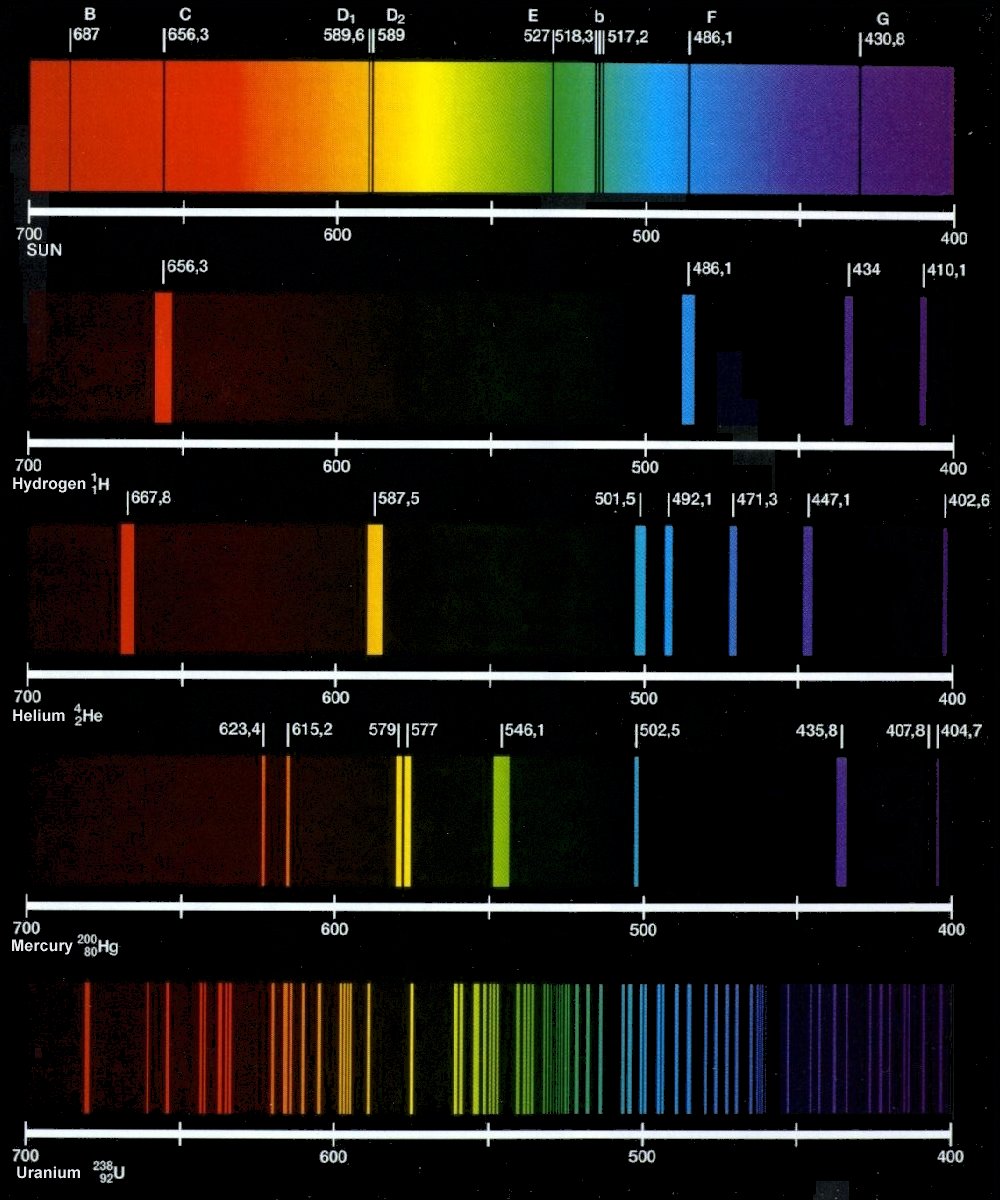
Types of emission and absorption spectra: ~ Pooza Creations

Radon - Home

Tests of Big Bang Cosmology - Week1

What are the spectral series we see in the Hydrogen atom emission

Enter Title Jeopardy Template

13.1: The Electromagnetic Spectrum - Chemistry LibreTexts

Light and Dark: Understanding Spectrometry | by Amelia Settembre | Medium

Absorption/emission lines (article) | Khan Academy
